|
Hydrogen Cars could be around the corner as
experiments onboard the International Space Station could accelerate
the drive toward a hydrogen-based economy.
by Dr Tony Phillips and Steve
Price
Imagine pulling up to a filling station,
inserting the nozzle into the tank and the gas flowing into your
tank is ... hydrogen. It's colourless, odourless and the byproduct
of burning hydrogen is water vapour, quickly and safely absorbed
by the environment. One pound of hydrogen supplies three times as
much energy as a pound of gasoline. And it's the most plentiful
element in the universe! No wonder scientists are trying to figure
out how to make hydrogen work as a practical fuel.
"Dozens of companies, including all
the major automobile manufacturers, have designed engines that burn
hydrogen - they're a lot like the internal combustion engines we
have in cars today," says Al Sacco, director of the NASA-supported
Centre for Advanced Microgravity Materials Processing (CAMMP) at
Northeastern University in Boston. "Fuel cells - another possible
source of power for cars - use hydrogen, too. To make these technologies
work in the real world, scientists must find a way to store and
transport hydrogen safely at a cost comparable to that of gasoline
which powers the cars we use
today."
It's not easy: Hydrogen gas is light
and elusive. Tiny H2 molecules like to sneak through
cracks and seals - and once free they quickly disperse. Hydrogen
diffuses four times faster than methane and ten times faster than
gasoline vapours. This is great for safety because a leak is quickly
diluted and rendered harmless. It's a headache for anyone who wants
to store the gas.

Credit and copyright: Fuel
Cell Today
A prototype
hydrogen fueling station in Las Vegas, NV.
|
Liquid hydrogen is more compact and
easier to contain, but it can be troublesome, too. Hydrogen liquefies
at a temperature of about 20oK (-253oC). Maintaining
a tank full of liquefied hydrogen requires a heavy cryogenic support
system, which may not be practical for passenger cars. Liquid hydrogen
is actually cold enough to freeze air. This could cause plugged
valves and unwanted pressure build-ups. Insulation to prevent such
problems adds to the weight of the storage system.
How can we overcome these obstacles?
Simple: put rocks in your gas tank.
Not ordinary rocks. Zeolites. Sacco
explains: "Zeolites are porous, rocky substances that act like molecular
sponges. In their crystalline form, zeolites are threaded by a network
of interconnected tunnels and cages, similar to a honeycomb." A
fuel tank lined with such crystals might be able to trap and store
hydrogen gas "in a liquid-like state - without heavy cryogenics."
With support from NASA's Space Product Development program at the
Marshall Space Flight Centre, Sacco and colleagues at CAMMP are
working to make zeolite gas tanks a reality.
The name zeolite comes from the Greek
words "zeo" (to boil) and "lithos" (stone), literally meaning "the
rock that boils." This is because zeolites give up their contents
when heated.
Sacco described how a temperature-controlled
zeolite gas tank might work: "We would add some negatively-charged
ions to the zeolite. These ions act like caps, just like caps on
an ink bottle; they block the zeolite's crystalline pores. By heating
the tank - just a little - we can make the ions move away from the
pores. We fill the zeolite with hydrogen, drop the temperature back
to normal, and the ions slide back in place, sealing off the exits."

Image credit and copyright: CamaroMuscle.com
The gas
tank of a Chevy Camaro. Automakers would like hydrogen fuel
tanks to be about the same size and weight - and hold the
same amount of energy.
|
Nearly 50 kinds of zeolites with different
chemical compositions and crystal-structures are found in nature,
and chemists have figured out how to synthesize many more. Anyone
with a cat has seen some: they act as odour-absorbers in kitty litter.
"The zeolites we have now can store quite a bit of hydrogen," notes
Sacco. "But not enough."
How much is enough?
Picture this: Your car's fuel tank
is lined with crystallized, porous rock and that "rock" weighs 93
pounds. You pull into a hydrogen fueling station and the attendant
forces 7 pounds of hydrogen into the zeolite-lined walls of the
tank. This, theoretically, would be the hydrogen equivalent to a
full tank of gasoline - in both total weight and energy content.
"If we can grow zeolite crystals that
hold 6% to 7% of their own weight in hydrogen," says Sacco, "then
a zeolite tankful of hydrogen would be competitive with an ordinary
tankful of gasoline." The best existing zeolites can hold only
2% to 3%, however.
In 1995, Sacco traveled to space as
a mission specialist onboard the space shuttle Columbia (STS-73).
His purpose: to grow better zeolite crystals. "In low-gravity, materials
come together more slowly, allowing zeolite crystals to form that
are both larger and more orderly." Zeolite crystals produced on
Earth are small, roughly 2 to 8 microns across. "That's about one-tenth
the thickness of a human hair." The ones he grew on the space shuttle
were not only 10 times bigger, but also better organized internally - a
promising start.
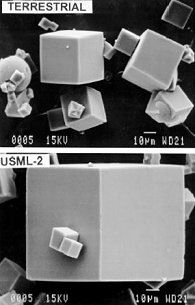
more
Zeolite
crystals grown on Earth (above) and zeolite crystals grown
onboard the shuttle Columbia in 1995 (below)
|
"The next step is the International Space
Station," says Sacco. He and others at CAMMP have built a Zeolite
Crystal Growth Furnace, which was installed on the ISS in early 2002.
"Ken Bowersox, the ISS Expedition 6 commander, has used the furnace
to grow some crystals for us. Ken had to correct some unexpected problems
with the mixing of the crystal growth solution - this shows the values
of humans in space - but after that the experiment went smoothly."
"Now we need to get those crystals back to Earth
where we can examine them. A few might come down in May," when the
Expedition 6 crew leaves the ISS in an Soyuz capsule. "I'd really
like to see them," says Sacco.
The goal, he says, is not to mass produce zeolite
crystals in space. That's not economical - at least not yet. "We
simply want to find out if it's possible to grow zeolite crystals
that can reach the 7% threshold. If we can do that in space, we'll
figure out how to reproduce the process on the ground."
Throughout his career, Sacco has envisioned
a worldwide transition from fossil to hydrogen fuels. It's a big
dream, but it could happen. "Zeolites may be the key to hydrogen
fuel as a leapfrog technology."
Coming soon… a hydrogen fueling station near
you?
|
