|
The high-tech rocket fuels of the future could
be made from a surprisingly low-tech material: candle wax!
by Patrick L Barry
Waiting inside his Mercury capsule
for the command that would start the countdown and make him the
first American in space, Alan Shepard yelled impatiently, "Let's
light this candle!"
Those words may turn out to be more prophetic than Shepard intended.
Since 2001, NASA's Ames Research Centre has been testing a new rocket
fuel made from - believe it or not - candle wax.
"We actually ordered the wax for our
test firings through a commercial Web site that sells candle wax
in bulk," says Arif Karabeyoglu, who developed the theory behind
paraffin-based rocket fuels and is currently a research associate
at Stanford University.
"We use the exact same wax found in 'hurricane' candles," he says.
Safer to handle and better for the environment than today's solid
rocket fuels, this modern twist on an ancient fuel could someday
propel sounding rockets and commercial-payload rockets into space.
It could even form the heart of a new generation of shuttle solid
rocket boosters (SRBs) that would have a key safety feature today's
SRBs lack: an "off" switch.

A material
in household candles, paraffin, could become the environmentally
friendly rocket fuel of the future.
|
This may seem a shockingly primitive
fuel for 21st Century rocket technology. After all, humans have
been burning candles (today often made of "paraffin" wax) for the
last five millennia. Why didn't someone think of using it for rockets
before?
As anyone who's lit a candle knows,
paraffin normally burns quite calmly, and it's difficult to make
it burn at all without a wick. By all appearances, it just wasn't
the kind of high-powered, explosive fuel needed to blast a rocket
off of the planet!
Working in collaboration with David Altman,
currently president of Space Propulsion Group, and Brian Cantwell,
a professor at Stanford, Karabeyoglu figured out a way to make paraffin
burn three times faster than had ever been achieved before - fast
enough to serve as rocket fuel.
More than 40 test firings by the Stanford-Ames
collaborative project have shown that the idea works as advertised.
That's good news for the rocket industry, because this paraffin
fuel would be much simpler and safer to work with than the toxic,
explosive fuels used today.
Just think of a household candle. You can safely carve it, melt
it, and mould it. If it's free from artificial colours or perfumes,
you could even lick it or chew on it. You could burn dozens of them
in a room with no fear of toxic gases making you sick.
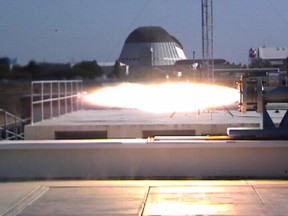
Image courtesy NASA
That's no candle flame! This test of the paraffin-based
fuel was conducted at NASA's Ames Research Centre
|
Don't try any of these things with conventional solid rocket fuels!
One reason for the benign nature of candle wax is that the oxidizer
needed for it to burn is separate from the wax itself: air in the
case of candles, and pure oxygen for rockets. (Chemically speaking,
combustion is the rapid "oxidation" of the fuel, usually by combining
with the oxygen gas in the air. That's why fires go out when deprived
of air.) This kind of rocket with a solid fuel and a separate gaseous
or liquid oxidizer is called a "hybrid" rocket.
In contrast, today's solid-fuel rockets use
solid materials such as perchlorate compounds as oxidizers, and
the fuel and oxidizer are mixed together before being packed into
the rocket. In other words, the fuel is "charged" and ready to explode
... not a friendly material to work with.
It's not friendly for the environment either.
When today's solid fuels burn, they produce the acidic compound
hydrogen chloride and other noxious chemicals. When it rains, these
compounds find their way into lakes and soils, and the increased
acidity can harm plant and animal life.
Paraffin, in contrast, burns cleanly. The only gases left behind
are water vapour and carbon dioxide. Rocket launches are still so
rare that the total pollution they emit is tiny compared to that
from cars, airplanes, and coal-fired power plants. But in the future,
as more countries and private companies begin launching people and
payloads into space, clean-burning rocket fuels will become an increasingly
important environmental issue.
Using hybrid rockets would make all these rocket
launches a bit safer as well.
By controlling the flow of the oxidizing
gas, "hybrid rockets ... can be throttled over a wide range, including
shut-down and restart," Cantwell said in a prepared statement. "That's
one reason why they could be considered as possible replacements
for the shuttle's current solid rocket boosters that cannot be shut
off after they are lit."

Image courtesy NASA
NASA and
Stanford scientists and engineers work on the testing rig
for the new paraffin-based solid rocket fuel. Pictured are
(clockwise from bottom-left): Brian Cantwell, Arif Karabeyoglu,
Shane De'Zilwa, Rusty Hunt, Dave Yaste, Kent Shiffer, Greg
Zilliac.
|
"A hybrid rocket equivalent to the
space shuttle's solid rockets would be about the same diameter,
but would be somewhat longer," Cantwell continues. "One design concept
being considered is a new hybrid booster rocket that is able to
fly back to the launch site for recharging," he says, which would
save considerable cost and time in preparing the boosters for the
next launch.
However, we won't be seeing paraffin-based shuttle
SRBs for many years to come, if ever, Karabeyoglu says. The technology
is still in the demonstration phase, and would likely be used for
years on smaller rockets before being considered for NASA's flagship
launch vehicle.
But if the tests continue to go well, the launch director at Mission
Control may one day mean it quite literally when she or he says,
"All right, enough waiting around ... let's light this candle!"
|
