|
Astronomers using the Chandra X-ray
Observatory are seeing how supernovae spray the essential elements
of life into interstellar space.
by Dr. Tony Philips
In order to make an apple pie from
scratch, you must first create the universe. Carl Sagan
Medical students toiling over
their homework in organic chemistry might be excused for day dreaming
about a universe without carbon or oxygen. University essentials like
spaghetti and baked beans would be unheard of in a carbon-deprived
cosmos, but even the toughest chemistry course would be a snap!
Such a universe existed about 10 billion years ago. According to the
Big Bang theory, just before the first stars formed the cosmos was
made entirely of the three simplest atoms: hydrogen (H), helium (He)
and small amounts of lithium (Li). It was every beleaguered chemistry
student's dream come true. There were no oxygen or carbon atoms or
any of the elements that make up the bulk of the periodic table today.
A quick glance around the room is proof that times have changed. Light
elements that filled the early universe are rare on Earth, while our
planet and we ourselves consist substantially of the heavier atoms,
like oxygen and nitrogen, which were missing at the dawn of cosmic
history.
What happened between then and now? Star formation.
|
The first stars that collapsed from primordial
gas clouds 10 billion years ago were made of hydrogen and helium.
As gravity caused these newborn stars to contract, temperatures near
their cores reached levels that triggered nuclear fusion, the same
process that powers our Sun today. In a stellar fusion reactor, lightweight
atoms like H and He are smashed together to form heavier species.
Hydrogen and helium is transmuted to elements like carbon, nitrogen,
and oxygen in the cores of mid-sized stars. Stars that are ten times
or so more massive than the Sun can assemble atoms as heavy as iron
(Fe) before they explode as cataclysmic supernovae. Atoms more massive
than Fe form in supernova blast waves (accounting for useful substances
like plutonium and uranium). The debris from supernovae seed interstellar
clouds with the raw materials for rocky worlds and carbon-based life.
In the immortal words of Carl
Sagan, "we are star stuff."
Scientists using NASA's Chandra X-ray Observatory are enjoying a close
up look at star stuff emerging from one of the youngest supernova
remnants in our Galaxy. Cassiopeia A - Cas A for short - is the remnant
of a star that blew itself apart about 9,400 years ago. It is relatively
close - only about 9,100 light years away - so it should have been
bright enough to cause a stir when it appeared in the night sky in
the mid-1600s, but most astronomers missed the explosion. Sir John
Flamsteed, Britain's Astronomer Royal, may have seen Cas A in 1670.
If so, he misidentified it as a star and made no follow-up observations.
No other sightings are known.
Cas A is very faint when viewed in visible light but its huge shell
(13 light years across) is filled with 30 million °K gas that glows
brilliantly at x-ray energies. The remnant is big, about one-sixth
the width of the full moon as seen from Earth, so it makes an attractive
target for the high-powered Chandra X-ray telescope.

The Cas A
Supernova inset against a picture of the moon to show their
comparative sizes.
|
Chandra carries an instrument called the Advanced CCD Imaging Spectrometer
(ACIS) that can measure the energy of incoming x-ray photons and associate
them with specific chemical elements. Using the ACIS, astronomers
are taking pictures of Cas A that reveal the distribution of heavy
atoms like oxygen, silicon and iron in the supernova's rapidly expanding
shell that show how those elements are mixing into the ambient interstellar
medium of gas and dust.
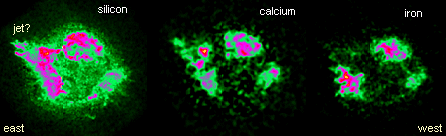
Recently, NASA released images of Cas A at x-ray wavelengths emitted
by ions of silicon (Si), calcium (Ca), and iron (Fe). On the eastern
side of the supernova's shell, Ca and Si images reveal a high speed
jet erupting into a relatively low-density region of the interstellar
medium. Scientists speculate that the jet might signify an asymmetry
in the original supernova explosion. On the opposite side, observations
at radio and other wavelengths indicate that Cas A is plowing into
an interstellar molecular gas cloud that confines the shell's outward
flow.
 NASA/GSFC/U. Hwang et al
NASA/GSFC/U. Hwang et al
Chandra X-ray
Images of the Cas A supernova remnant
|
There are intriguing differences between
the maps of Ca and Si and the map of Fe, which is clumpier and does
not show the jet so clearly. Material rich in iron comes from the
inner core of the star where fusion temperatures were highest. Scientists
have examined these maps carefully and note that iron-containing
knots from deepest in the star seem to be nearest the outer edge
of the remnant. This means they were flung the furthest by the explosion
that created Cas A.
Cas A's outer envelope is expanding
at 800 km/s (about 1.73 million mph). That's rapid enough that images
taken by Chandra over the years will show how knots in the shell
change and cool. By monitoring these changes, Chandra scientists
hope to learn more about how quickly and in what form different
elements are deposited into the interstellar medium.
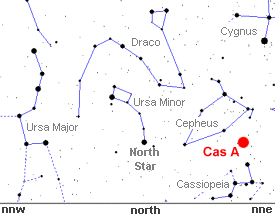
Cassiopeia
A is too faint to view with the unaided eye, but it's easy
to see where the remnant is in the northern summer sky. Simply
go outside just after sunset and look 45 degrees above the
north-north east horizon between the constellations Cepheus
and Cassiopeia.
|
Even after more than 10 billion years
of star formation, hydrogen and helium still are overwhelmingly the
dominant atoms in the cosmos. Heavier atoms like the ones we see in
the shell of Cas A are over represented on Earth because H and He
are volatile gases that solar heating drives from the low-gravity
terrestrial planets. Massive Jupiter, on the other hand, is made up
almost entirely of hydrogen, as is the Sun.
Heavy elements may be no more than
rare cosmic pollutants, but they are exceedingly important to us.
Without them, solid, rocky planets would be impossible, and the
prospects for Earth-like life would be correspondingly dim. As it
is, the iron we see now in Cas A might one day flow as hemoglobin
in the blood of some future alien species. Fast moving knots of
silicon from the supernova could provide the raw material for sand
on otherworldly shores, where crashing waves of H2O send
thunderous sound waves through a nitrogen-rich atmosphere. And just
perhaps, on that fanciful alien world, hardworking science students
distracted by the beckoning sounds of distant waves might wish for
less organic chemistry and more time on the beach.
|
