|
On a planet that's colder than Antarctica
and where water boils at ten degrees above freezing, how could liquid
water ever exist? Scientists say a dash of salt might help.
by Dr Tony Phillips
When scientists revealed dramatic new
pictures of flood-like gullies on Mars, the big surprise wasn't
that the Red Planet might harbour water. Researchers have known
for years that water exists there. There are trace quantities of
water vapour in Mars' atmosphere and substantial amounts
of water ice at the martian poles. There may even be enough
frozen water beneath Mars' surface to fill a large ocean if melted.
What was amazing is that water may be present as a liquid
very near the planet's surface and occasionally on top of the surface
when underground deposits burst forth for a brief flash flood.
"We have conditions on Mars that seem
to forbid liquid water very close to the surface," said Michael
Carr of the US Geological Survey (USGS) at a press conference in
June 2000. "At high latitudes, where the gullies are located, the
temperatures are 70 to 100 degrees centigrade below freezing. It's
incredibly cold. We expect the ground to be frozen 3 to 6 km deep."
The low temperature of Mars conspires
with the planet's thin atmosphere (it is 100 times thinner than
Earth's) to make water possible in only two forms: solid ice and
gaseous vapour. A cup of liquid water transported Star Trek-style
to the surface of Mars would instantly freeze or boil (depending
on the local combination of temperature and pressure). Researchers
think that the water which carved the martian gullies probably boiled
explosively soon after it erupted from underground.

more from GSFC
Martian gullies in Newton Crater. Scientists hypothesize
that liquid water burst out from underground, eroded the
gullies, and pooled at the bottom of this crater as it froze
and evaporated. If so, life-sustaining ice and water might
exist even today below the Martian surface - water that
could potentially support a human mission to Mars.
|
"The air pressure is so low on Mars
that even in the most favourable spots, where the pressure is higher
than average, liquid water is restricted to the range 0 to +10 °C,"
says Bob Haberle of the NASA/Ames Research Centre."Fresh water on
Mars begins to boil at 10 °C. Here on Earth we can have water anywhere
between 0 and 100 °C - that range is reduced by a factor of ten
on Mars."
If the thought of boiling water at
10 degrees °C seems bizarre, simply consult a high-altitude cookbook
for a reality check. On mountaintops where the air pressure is low,
water boils at a lower temperature than it does at sea level. (At
9000 ft a 'three-minute' boiled egg takes about five minutes to
fully cook!) Mars simply takes the principles of high-altitude cooking
to an extreme.
Although any liquid water exposed to
Mars' low-pressure atmosphere is likely to boil, vapour is not the
most important repository of martian H2O. If all the
vapour in the present-day atmosphere rained down on one spot, it
would barely fill a small pond. On the other hand, the martian poles
contain lots of water in the form of a solid. The north polar cap,
composed primarily of water ice, is 1200 km across and up to 3 km
thick in some places. The water volume there is about 4% of the
Earth's south polar ice sheet. Even more water ice is thought lie
deep underground.

more
from GSFC
Water on
Mars.
A: A 3D view of the Martian north
pole created from Mars Global Surveyor laser altimeter data.
The cap is composed mainly of solid water ice.
B: Wispy clouds of water ice hover
over the Kasai Vallis region of Mars.
C: Ground frost (or snow) consisting
of water ice at the Viking 2 landing site on Utopia Planitia.
|
So, the big question is not whether
water exists on Mars - it does - but rather is there liquid water
despite the planet being so cold? The prospects for life on Mars,
both human and martian, hinge on the answer.
"First of all, you have to remember that the average atmospheric
pressure on Mars is very close to the triple point of water," explains
Richard Hoover, an astrobiologist at the Marshall Space Flight Centre."You
only have to increase the pressure a little bit to make liquid water
possible."
The 'triple point' is the combination of pressure (6.1 millibars)
and temperature (0.01 °C) at which water can exist simultaneously
in all three states: a solid, a liquid and a gas. On Earth, our
experience with the triple point is usually limited to ice skating.
The temperature of ice on a skating rink is just a fraction of a
degree from the triple point. A little bit of pressure on the solid
ice can cause it to transform to a liquid. The weight of a skater
applied to the ice along the blade of the skate therefore creates
a thin layer of liquid water that lubricates the blade and makes
gliding possible.
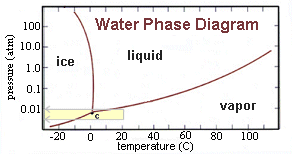
A phase
diagram of water. The 'triple point' (labeled "C" in the
diagram) is the temperature and pressure where all three
types of water can exist at once. In the diagram, note that
liquid water cannot exist below 6.1 millibars. This fact
is significant because the atmospheric pressure at the martian
surface hovers just below that value. Any water that might
form on a warm afternoon from melting water would quickly
disappear in the desiccated martian atmosphere.
|
On Mars the globally-averaged surface
pressure of the planet's atmosphere is only slightly less than 6.1
millibars.
"That's the average," says Haberle, "so some places will have pressures
that are higher than 6.1 millibars and others will be lower. If
we look at sites on Mars where the pressure is a bit higher, that's
where water can theoretically exist as a liquid."
The atmospheric surface pressure on Mars
is remarkably close to the triple point pressure 6.1 millibars.
Is that a coincidence? Some scientists think not. If the global
pressure were higher and liquid water was widespread on Mars's surface,
CO2 in the atmosphere would dissolve in water and react with silicate
rocks, trapping atmospheric carbon dioxide in carbonate minerals.
This process would thin out the atmosphere until the pressure dropped
below the triple point. Thus, the martian atmosphere could be self-limiting
in this respect. [more information]
Haberle has developed a sophisticated
climate model for Mars based in part on Mars Global Surveyor topography
data. A simple version of the model is the basis for daily martian
weather forecasts at the Ames Mars
Today web site.
"I used the model to look for regions that meet the minimum requirements
for liquid water - above the triple point and below the
boiling point," explained Haberle. "According to the model, the
highest surface pressure, 12.4 millibars, occurs at the bottom of
the Hellas Basin (a low-lying area created by an ancient asteroid
strike). The problem is that the boiling temperature there is only
+10 °C. It can't get very hot or the water will boil away."
Evaporation of water in contact with Mars' dry atmosphere is also
a problem, says Haberle. "Liquid water can be stable against freezing
and stable against boiling, but unstable with respect to evaporation.
The situation is analogous to Earth's oceans. Liquid water on the
surface does not freeze ... or boil, yet it can evaporate if the
atmosphere is not saturated with water vapour.
"There are 5 five distinct regions where we might sometimes find
surface water: in the Amazonis, Chryse and Elysium Planitia, in
the Hellas Basin and the Argyre Basin. Together they comprise about
30% of the planet's surface. That's not to say that liquid water
really does exist in those places, just that it could."
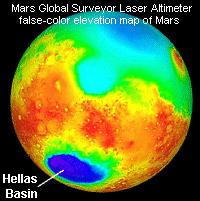
The massive
Hellas impact basin in the southern hemisphere of Mars is
nearly 9 kilometres deep and 2,100 kilometres across. The
air pressure at the bottom of the basin is about twice the
global average. In this false-color image based on measurements
from the Mars Global Surveyor laser altimeter, red and white
colours denote high elevations and blue denotes low.
|
Conditions would be favourable for
liquid water only during the martian day. The temperature falls
precipitously at night, so any liquid would re-freeze. At the Viking
lander sites, for example, instruments registered temperatures as
high as -17 C in the air and +27 °C in the soil on sunlit summer
days. After sunset, thermometer readings plunged back to -60 °C
or below. [click for more
information about martian temperatures]
"One thing we have to be careful of
is our everyday experience that water always freezes at zero degrees,"
noted Hoover. "It doesn't. Water containing dissolved salts freezes
at a significantly lower temperature. Don Juan Pond in Antarctica
is a good example. It's a high salinity pond with liquid water at
temperatures as low as -24 °C."
"Salts have the potential to significantly lower the freezing point
of water," agrees Steve Clifford of the Lunar and Planetary Institute.
"Indeed, there are some combinations of salts that can lower the
freezing point by as much as 60 °C. However, thermodynamic and chemical
stability arguments (arising from work by Benton Clark) suggest
that, on Mars, the most potent freezing point-depressing brines
are likely to be based on NaCl (common table salt)."
An analysis of a Martian meteorite
by Arizona State University scientists suggests that ancient martian
oceans - if they existed - contained a mix of salts similar to
those in Earth's oceans today. That wasn't the first clue that Mars
was salty, though. In 1976 the two Viking landers analyzed martian
soil and found that it probably contained 10 to 20 percent salts.
Martian rocks, like those on Earth, react to form salt and clay
minerals when exposed to water. On our planet this process gives
rise to a variety of brines in the western salt lakes of North America.
The detailed chemistry of the brines depends on the composition
of local rocks.
Another way to help keep water liquid
- on Mars or Earth - is to keep it moving.
"If you know a hard freeze is coming where you live, what's the
first thing you do?" asks Hoover. "You turn your faucets on a little
to let water trickle out. This way your pipes won't freeze."
The same principle applies on Mars where salty water could be moving
through subterranean aquifers. "Ice is a crystal," explains Hoover,
"and it's harder to form crystals when the water is flowing."

Photos Courtesy Richard B. Hoover
Sampling
ice from a moulin in the tongue of Alaska's Matanuska glacier.
Orange moss can be seen growing on broken rock debris on
ice ledge
|
Hoover visited the Matanuska Glacier
in Alaska to search for cold-loving microorganisms living in and
around the ice.
"I chose the Matanuska Glacier to visit
because it's accessible and has dark rock in contact with ice,"
says Hoover. "The sun shining on the rock causes the ice to melt.
There are pools of liquid water where microorganisms grow in abundance.
There is something very interesting and exciting about this picture
of me taking samples from the edge of a moulin (a water-carved crevasse).
Most of what we see is ice and the air temperature is below freezing,
yet there is liquid water pouring out of the glacier. How is that
possible? The water had broken free further back up the glacier
where sunlit rocks melted the ice. Then it flowed beneath the ice
until it broke through a hole in the wall of the ice. Everything
the liquid water came in contact with was freezing, yet the moving
water did not freeze.
"I have also seen liquid water running from snow melting on dark
rocks heated by sunlight in Antarctica, even though the air temperature
was below -20 °C."
There are many places on Earth where
liquid water and ice co-exist in sub-zero conditions, says Hoover.
The most famous example is Lake Vostok, an expanse of water roughly
the size of lake Ontario lying 4 km beneath the Antarctic ice sheet.
The ice sheet acts as a blanket, shielding the lake from Mars-like
temperatures at the surface.
Will explorers one day discover oases like Lake Vostok beneath icy
terrain on Mars? No one knows. But instead of "Follow the Water,"
the mantra of future colonists on the red planet might well be "Follow
the Salt."
FirstScience Editorial on 'Missions
to Mars'
|
