|
Altered gravity plays an unexpected
role in obesity and weight loss.
by Karen Miller and Dr Tony
Phillips
Astronauts have long
known that space travel is a good way to diet. The excitement of
launch. Thrilling vistas seen from Earth orbit. Floating weightless.
Maybe a touch of motion sickness. Who can eat at a time like that?
Rats, apparently, feel
the same way. Rats in space (they've been there onboard the space
shuttle) also under-eat. They grow lean compared to rats on Earth.
Curiously, rats experiencing high gravity (inside gently-spinning
centrifuges) under-eat, too. And this suggests there's more to the
story than thrilling vistas:
"Altered gravity somehow
disrupts the natural ability of animals to maintain their own weight,"
says Barbara Horwitz, a professor of physiology at the University
of California. No one understands exactly why that should be, but
it's probably an important clue to the inner workings of weight
control - something that interests people on Earth just as much as
astronauts in space. Horwitz is studying the phenomenon in rats
at her laboratory in Davis, California.
Although some of us
who struggle with weight issues may find it hard to believe, animals,
including humans, have evolved a complicated system for maintaining
appropriate weight. You'd expect that: the bodies of animals that
are too heavy, or not heavy enough, don't function properly.

Astronaut Loren Shriver eats M&Ms onboard the space shuttle
Atlantis.
|
Feeding behaviour is
essential, not only to the health of individuals, but also to the
survival of whole species. The body stores energy in fat, and there's
a minimum amount an organism must have before it can get pregnant.
"Animals that lose a lot of fat don't reproduce," says Horwitz.
But the complex network
that signals when to eat and when to stop eating can go awry. This
could be a contributing cause of, e.g., the "obesity epidemic"
in the United States, under-eating among astronauts, and maladies
such as the "wasting syndrome" linked to AIDS.
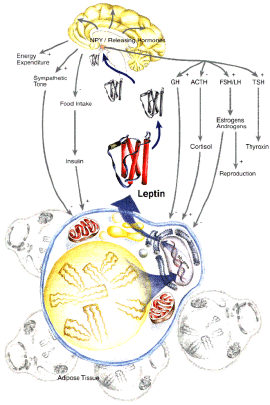
Horwitz is particularly
interested in leptin regulatory pathways. Leptin is a hormone that's
key to regulating appetite: when it was first discovered in the
mid-1990's it was regarded as a possible way to treat obesity in
humans. Leptin is produced by fat cells. The more fat cells an organism
has, the more leptin circulates through its body.
Leptin manages appetite
by activating receptors in the hypothalamus, a part of the brain.
These receptors control the production of small signaling proteins
called neuropeptides. Leptin increases the amount of neuropeptides
that make you feel full, and decreases the amount of neuropeptides
that make you feel hungry.
Horwitz is studying
leptin regulatory pathways in rats: The animals live in a 2-g
(twice normal gravity) centrifuge in individual, free-swinging cages,
for as long as eight weeks. Even though they're working against
twice the gravity they're used to, the rats don't seem to mind.
They move around, they groom themselves. If they're allowed, they'll
even breed on the centrifuge, says Horwitz.
Living in double gravity
naturally requires more energy. The rats were offered all the food
they wanted, yet, at first, they ate less than they needed to maintain
their body mass - much like astronauts in low gravity.
Horwitz and colleagues
tested the rats (along with 1-g control groups) at 1, 2
and 8 weeks. During the first week, some of the rats' neuropeptides
were mixed up. One, in particular, which stimulates feeding and
therefore should have increased, actually went down.
By the eighth week,
things were back to normal - almost. The animals produced the same
amount of neuropeptides in both 1-g and 2-g habitats.
Double-gravity rats were finally eating as much as they needed.
But they remained lean: they never regained the fat they lost at
the beginning of the study.
"That means that the
pathway somehow was changed," says Horwitz. "The relationship between
the amount of fat, and how much leptin was secreted, and the functioning
of the feedback system is altered in high gravity."
Copyright:
Mediagnost. All rights reserved.
An
artist's concept of the brain-body appetite control system.
|
Horwitz hopes to pinpoint the exact
mechanisms by further testing the rats' genes: Each neuropeptide
in the appetite feedback loop is produced or "expressed" by a gene
that has been activated. Using a technology called DNA microarrays,
Horwitz and colleagues examine thousands of rat genes at a time.
They can see which genes have been activated, and how active they
are.
Understanding the chemical
pathways at this basic level could lead to "countermeasures,"
i.e., treatments to restore broken leptin regulatory systems.
Many researchers now
believe that leptin's main role in humans is protecting against
weight loss more so than weight gain. It makes sense: food surpluses
are a relatively new phenomenon. Humans have evolved to withstand
deprivation, not excess.
This makes leptin,
potentially, even more important to astronauts: It's part of a regulatory
pathway that keeps them from becoming too lean when stress, motion
sickness and bland food take away their appetites.
Horwitz's research
is important here on Earth, too. People with weight control problems
like obesity may have defective leptin regulatory pathways: they
tend to have plenty of leptin coursing through their bodies, but
it does not cause them to eat less. The big question is why. Maybe
their leptin receptors don't work well, or their neuropeptides aren't
produced properly. Or it could be something else entirely.
Somewhere, along the pathway less travelled,
lies the answer.
|
