|
Upcoming experiments planned for the International
Space Station will help engineers on Earth learn to handle undercooled
fluids.
by Patrick L. Barry
High-performance golf
clubs. Ultra-sharp knives. Superior fiber optics for telecommunications.
Tough, lightweight materials for future spacecraft.
What do all these things
have in common? They can all be made using "undercooled" liquids:
molten materials that are cooled below the normal freezing point
yet, through special handling, are kept in a liquid state.
By avoiding normal
freezing, one can coax the liquid into becoming a very different
kind of solid. In normal freezing, the molecules of the liquid settle
into an orderly crystalline grid, like soldiers falling in line.
This is how ice, normal metals, and indeed most solids are formed.
Undercooled liquids solidify in a different way. As they cool, they
thicken and eventually stop flowing - like a liquid "on pause."
The result is a solid whose molecules remain scrambled in a semi-random,
amorphous arrangement. This molecular structure, most commonly found
in window glass but possible in metals, too, has special properties.
Amorphous metal alloys, for instance, can be twice as strong and
three times more elastic than steel.
There's great potential
for products made from these liquids, but they are notoriously difficult
to handle.
An undercooled liquid
is a delicate, unstable state of matter. It desperately "wants"
to crystallize into a normal solid. All that's needed is a place
for the crystallization to begin - such as the crystalline surface
of a container wall or even a speck of dust - and the liquid will
suddenly freeze solid. In other words, working with undercooled
liquids is a bit like juggling mousetraps: they're prone to suddenly
"snap" and ruin the trick.

Image courtesy Liquidmetal Technologies.
A few
of the things manufacturers can make better using undercooled
fluids.
|
Remarkably, manufacturers
on Earth have managed to make some products from these liquids anyway:
computer components, golf
clubs, tennis racquets. There's even a solar wind collector
on board NASA's Genesis spacecraft made of undercooled amorphous
metal.
These items are just
the beginning. As engineers learn more about the basic physics and
properties of undercooled fluids, they'll be able to do more with
them. And that's where the International Space Station (ISS) can
help. In the weightlessness of Earth orbit, it's possible to study
fluids without holding them in containers that might trigger premature
crystallization.
Edwin Ethridge, a materials
scientist at NASA's Marshall Space Flight Centre, and Prof. William
Kaukler of the University of Alabama in Huntsville are working on
a way to measure the viscosity of containerless fluids onboard the
ISS. Their idea is simple: If two floating drops of a liquid touch
each other, they will merge to form a single, larger drop. The speed
of this merger is partially controlled by viscosity - water will
merge much faster than honey, for example. So watching this speed
lets scientists measure the liquid's viscosity.
Good viscosity measurements
are critical for working with undercooled fluids, which thicken
dramatically as they cool. The friction between molecules in one
of these cooling fluids can skyrocket by as much as a quadrillion
times (1015) as it solidifies. Without a graph plotting
how this thickening occurs in relation to cooling temperatures,
engineers can't easily mould these liquids into useful shapes.
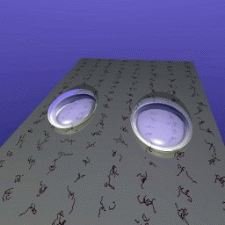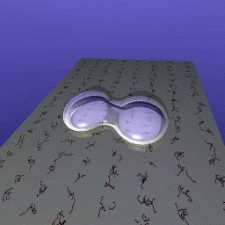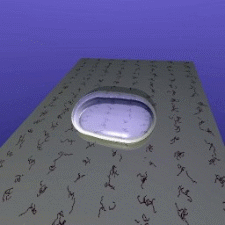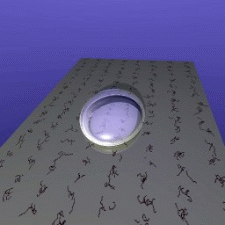
The speed at which droplets merge depends on their
viscosity.
|
To understand why, just imagine what
would happen if you designed a mould with lots of complex nooks
and crannies so that it works well for undercooled liquids with
the thickness of vegetable oil. But as you poured the undercooled
liquid into the mould, it cooled slightly, causing an unexpected
thousand-fold thickening - rendering the liquid as thick as honey.
The object produced is likely to look more like modern art than
a saleable product.
Getting the data to
make viscosity vs. temperature curves is the ultimate goal of Ethridge
and Kaukler's research. Their upcoming experiment, called Fluid
Merging Viscosity Measurements (FMVM), is a proof of concept. It
will show how viscosity measurements of containerless fluids can
be made in the microgravity environment of the ISS.
The physics is hard
enough, but the scientists had to tackle another problem as well:
Because room for sending research equipment up to the station is
limited while the shuttle fleet is grounded, the researchers had
to find a way to do their experiment using things that can be tucked
inside a Russian Progress supply rocket or found already onboard
the station.
"I have selected 8
liquids for testing," says Ethridge. "They've been loaded in syringes
that will be launched on a Progress rocket to the space station."
One of them is ordinary honey. Although it only crystallizes very
slowly, honey is actually an undercooled liquid. It works just fine
for proving that this "floating drop" method can accurately measure
a liquid's viscosity.
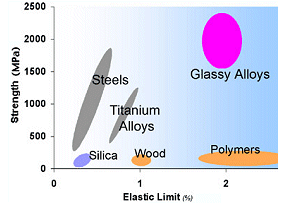
The strength
and elasticity of amorphous solids ("glassy alloys") exceed
that of many other materials.
|
The experiment goes like this: Honey
(or one of the other liquids) will be squeezed from its syringe
and transferred onto thin strings. "Nomex thread and string is available
on the space station and can be used to confine and control the
liquid drops in orbit. Thin solder wire may also be used to manipulate
the drops," notes Ethridge. With a drop clinging to each of two
strings, a crew member will bring them slowly together, allowing
the drops to gently touch and merge. A video camera kept aboard
the station will record what happens as the drops slowly form a
peanut shape and eventually a single sphere.
Back on the ground,
researchers will examine the footage frame by frame to determine
exactly how fast the drops merged. Because the viscosity of the
test samples is already known, researchers can compare the measured
value with the real value to see if they're on the right track.
The researchers currently
plan to conduct the FMVM experiment sometime during Expedition 8,
which is scheduled to begin in late October 2003. Their work could
result in a new way of knowing the viscosity of undercooled liquids.
And after that... no one knows, but golf clubs and kitchenware are
probably just the beginning.
|
