|
Scientists are learning how to use carbon
dioxide - the main gas in Mars' atmosphere - to harvest rocket fuel
and water from the red planet.
by Karen Miller and Dr Tony
Phillips
When astronauts first
go to Mars, it will be difficult for them to bring everything they
need to survive. Even the first tentative explorations could last
as long as two years - but spaceships can only carry a limited amount.
"We might have to do
what explorers have done for ages: live off the land," says chemical
engineer Ken Debelak of Vanderbilt University.
Explorers on Earth
could usually count on finding what they needed. The animals might
be strange, but they'd be there, and they'd be edible. Mars is barren.
But the challenge is the same. Astronauts will want to pull what
they need from the planet itself. And although that goal seems improbable,
Debelak believes it can be achieved. He is working on a NASA project
to make it happen. The key, he says, lies in the Martian atmosphere.
It's a meager atmosphere,
compared to Earth's, and it's about 95 percent carbon dioxide (CO2).
But that turns out to be an advantage. The carbon dioxide, says
Debelak, can be used to harvest almost everything else.

A painting by Paul Hudson
Exploring
Mars
|
Inside martian rocks
and soil lies a bounty of useful elements: magnesium and hydrogen
for rocket fuel, oxygen to breathe, water to drink. What's needed
is a solvent to get them out, and that's where the carbon dioxide
comes in handy.
"When CO2
is compressed to a pressure of 73 atm and heated to 31.1 degrees
Celsius, it becomes a supercritical fluid - and a marvelous solvent,"
says Debelak.
A supercritical fluid
is a high-pressure, high-temperature state of matter perhaps best
described as a liquid-like gas. Almost anything can become supercritical.
Water, for instance, becomes a supercritical fluid in the high pressures
and temperatures of steam turbines. Ordinary water is a good solvent.
Supercritical water is a great solvent - maybe even a little too
good. It dissolves the tips of the turbine blades.
Supercritical carbon
dioxide behaves much the same. CO2 molecules flow into
solid matter, surrounding atoms, pulling them apart and away.
more
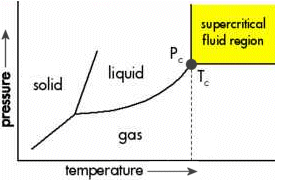
A phase
diagram. The critical point, denoted by the big gray dot,
is a special combination of temperature (Tc=31
C) and pressure (Pc=73 atm) where CO2
has properties of both liquid and gas. Above the critical
point, it becomes a supercritical fluid.
|
On Earth, supercritical
CO2 is not used much to dissolve things because there
are less expensive, more effective solvents close at hand. It is,
however, used to remove the caffeine from coffee beans, and sometimes
to dry-clean clothes. On Mars, Debelak believes, supercritical CO2
will play a much more important role.
For example: Magnesium
can be dissolved quite easily by supercritical CO2, Debelak
has found. "That's an experiment that we're quite excited about
at the moment," he says. Magnesium, which is likely to be found
in martian soil, ignites easily and can be used to fuel rockets.
In fact, says Debelak, one Mars exploration scenario called for
a lander to be made of magnesium - "the legs and so on." When the
astronauts were ready to go home, "you could chop it up, pack it
into a rocket engine, and then add some other oxidizer to fire it
off." Using CO2 as a solvent, magnesium could instead
be harvested directly from Mars.
Supercritical CO2
might also be used to generate water. Certain martian rocks (like
some of Earth's rocks) contain hydrogen. When these rocks are submerged
in supercritical carbon dioxide, a chemical reaction takes place.
The CO2's carbon becomes "fixed" in the rock, leaving
the oxygen free to find another partner: hydrogen. "The process
kicks out water," marvels Debelak. "You can actually use it to form
water."

more
A chamber
containing two phases of carbon dioxide - liquid and gas.
As temperature and pressure increase (from left to right),
the two phases merge to become a supercritical fluid.
|
Pulling water from rocks will probably have the biggest payoff, at
least in the short term, says Debelak. In addition to drinking, "you
can split water into hydrogen for fuel, and oxygen for breathing -
or as an oxidizer for some sort of engine." Eventually, colonists
could set up plants that use CO2 from the martian atmosphere
to process hundreds of kilograms of raw material a day.
A supercritical fluid
has some advantages over other solvents: Its solubility changes
dramatically when you alter the temperature or the pressure. You
can control it, so that sometimes it's a solvent for a particular
substance, and sometimes it's not. That makes it easy to recover
the material that has been dissolved. Let's say you have caffeine
dissolved in supercritical carbon dioxide. To recover the caffeine
(caffeine recovered from coffee beans is often put in soft drinks),
you just lower the pressure of the CO2 and the caffeine
drops out.

more
The rock-strewn
terrain around NASA's Viking 2 landing site on Mars.
|
Currently, Debelak
is trying to pin down the way a variety of substances behave in
supercritical CO2. He's looking at which minerals are
easily soluble and which are not. And if they're not, he's trying
to determine how their solubility can be improved. Adding other
substances to the CO2 sometimes helps, he says.
Debelak's work could
be useful on Earth, too. Carbon dioxide is often spotlighted because
of its damaging role in global warming. But as a solvent, it's benign.
Many solvents common in industry are toxic. They cause cancer, and
if they get into the water system, they stay for a long time. So
there's interest, says Debelak, in learning how to use CO2
as a 'green' alternative.
Carbon dioxide plays
widely different roles on Earth and on Mars. "That's what's intriguing,"
points out Debelak. "Mars is a totally foreign environment to us.
The rules are different."
"So that's what we're
doing - trying to figure out the rules," he says. "And then we can
figure out how to play the game ... on both planets."
Click
Here for a Special Mars Fact File
on FirstScience.com
|
