|
Diamonds,
rubies and sapphires captivate us by their symbolic values of wealth
and power, and also by their heavenly beauty. But these gems are
born in hellish fires deep below Earth’s surface.
by Hugh Williams
Look at the diamond in your ring, and it’s not only the most
valuable stone you’re likely to ever handle. It’s also
the oldest; it’s come from deeper in the Earth than any other;
and it’s the rock that has literally been through Hell…
Diamonds can’t
form on the surface of the Earth. The ultimate gemstone is actually
unstable at the pressures we live in. Diamond is made from pure
carbon, and the diamond in your ring is trying to change to graphite,
the form of carbon that’s most stable in our environment –
and best known as black pencil “lead”. Fortunately,
the transition takes place at an infinitesimally slow pace.

Beauty
and the beast: diamonds were born from tortured rocks
100 miles down
|
There’s only one place that diamonds can be
forged – deep beneath our feet, at least a hundred miles down.
That’s as far below us as the Space Shuttle orbits above us;
and far deeper than any borehole ever drilled. Down here, we are
truly in Hell: the pressure is 100,000 times higher than the pressure
of air around us, and the temperature is around 1000C.
The story of diamonds begins three billion years
ago, when the Earth was still young. After its fiery birth, the
surface of our planet solidified – not into a single uniform
piece of crust, but into several continental blocks. Each block
was shaped like a ship, with a solid central “keel”
reaching a hundred miles deep into the hot viscous rocks of the
Earth’s mantle.
Within the keel, there was a unique environment.
It was relatively cool and stable, amid the hot and ever-mobile
mantle rocks. Water, carbon dioxide and methane were dissolved at
high pressure in the mantle. They seeped into the keel, and reacted
together. The carbon precipitated out. Under the intense pressure
down here, the most stable form of carbon was diamond.
Nowhere else on Earth – nor at any other time
– could diamond form so readily. After this period, continents
never again formed such deep keels: instead, these rocky ships started
to drift around the globe in the process of plate tectonics. Today,
the bottoms of the oldest continents still carry their ancient load
of gemstones. The roots of Australia, Canada and southern Africa
are studded with a richness of diamonds that surpasses imagination.

Rough diamond –
the giant Cullinan stone as found in 1905
|
Fortunately
for diamond-lovers and geologists alike, some of these riches have
been blasted to the surface, in unique kind of eruption that has
its roots far deeper than any conventional volcano.
Through cracks
in the deep crust, lava has gradually seeped upwards, carrying diamonds
as passengers. This process should spell doom for the diamonds.
The pressure is dropping all the way, and by the time it reaches
the surface, reactions within the hot lava should turn the diamonds
into graphite, the form of carbon that’s stable at low pressure.
Diamonds can
only survive if they are spewed out in the surface suddenly, so
that they “freeze out” in their unstable form. And,
by a miracle of Nature, that’s exactly what has happened in
southern Africa and Australia.
Here, the diamond-rich
lava is also full of carbon dioxide gas. Once it’s within
two miles of Earth’s surface, the pressure of gas is higher
than the weight of overlying rock. Exactly like a shaken bottle
of champagne, the carbon dioxide erupts upwards. It punches a narrow
“pipe” up through the rocks, and bursts through onto
the surface, spraying out rocks and diamonds.
Today, we find
these pipes filled with bluish kimberlite rock, with diamonds scattered
throughout it. The diamond mines of South Africa – and now
Australia – provide an invaluable commodity not only to jewellers
and industry, but also to geologists. The diamonds give us a unique
insight far into the Earth, not just in the form of the gem itself,
but because some diamonds carry tiny fragments of unusual rocks
with them, our only samples of the planet from so deep beneath our
feet.

The
621 gramme Cullinan diamond was divided into 11 pieces
|
Rubies
and sapphires
Throughout history, rubies and sapphires have had
their own separate folklore and attributes. But, astoundingly, they
are actually the same gem – and identical to black crystals
of emery, used on an emery board for filing your nails. The only
difference lies in slight traces of impurities.
Corundum is the name that covers all these minerals.
It’s a tightly bound array of aluminium and oxygen atoms.
These two reactive elements bind together with immensely strong
chemical bonds, making corundum the second hardest substance after
diamond. In its most impure and cheap forms, such as emery, it’s
a vital abrasive for industry.
A crystal of
pure corundum would be colourless. But even the tiniest dash of
an impurity imparts a vivid colour. If the crystal picks up traces
of chromium, it glows with the deep red of a ruby. Any other ingredient,
tinting the gem with another colour, gives us a sapphire. Though
we think of sapphires as blue, they can range in colour from yellow
to green and purple. The rare Padparadsha (“lotus flower”)
type is a lovely orange sapphire.

Not
a ruby but a rare orange sapphire
|
These wonderful
gems are found in only a few locations on Earth, largely in a band
that stretches around the Indian Ocean from Madagascar and Kashmir
to Burma and Thailand. Australia and Montana also weigh in with
sapphire deposits.
Aluminium and
oxygen are among the commonest elements in our planet, so it may
seem odd that rubies and sapphires are so rare. But geology is never
simple. Silicon is also a major element in the Earth. If there are
silicon atoms around, the aluminium and oxygen atoms will pull them
into the molecule as well, to make minerals (like feldspars) that
are more complicated, but less beautiful and valuable.
So the search
for rubies and sapphires becomes the search for crucibles in the
Earth where silicon is missing. The raw material is often the debris
from granite outcrops that have been weathered away. The process
washes out compounds of silicon compounds, leaving an insoluble
white clay - a soft compound of aluminium oxide and water. It’s
called bauxite, after a huge deposit found at Les Baux in France.
In Australia and Thailand, ancient deposits of bauxite
have been caught up in the upheaval of plate tectonics. The bauxite
has descended over fifty miles into the Earth. Here, the rock is
at squeezed at thousands of atmospheres of pressure, in temperatures
of hundreds of degrees. Water is driven out of the bauxite, and
the aluminium and oxygen atoms are crushed together to form corundum.
Tiny traces of other atoms create the whole panoply of sapphire
colours, from blue through to yellow.
But the world’s biggest hoards of ruby and
sapphire come from more complex geochemical cauldrons.
In the jungles of Burma lies the deep valley of
Mogok. From time immemorial, local people have known it’s
the source of magnificent rubies. Yet there was a problem. The valley
is full of lethal snakes. The Burmese harvested the rubies by throwing
down hunks of meat. Rubies became embedded in the meat; birds of
prey carried off the meat and devoured it; and the local people
gathered the rubies from the birds’ droppings!
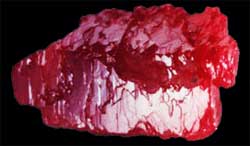
Ruby in its natural
state
|
In Mogok, a
seam of lava has pushed through limestone that was laced with specks
of aluminium oxide, and a smattering of chromium. The lava’s
heat melted the limestone into marble. The aluminium oxide in the
marble was concentrated into corundum crystals tinged with distinctive
chromium red. The Burma rubies known as “pigeon blood”
are the finest in the world.
The world’s
best sapphires are found in Kashmir – a “quintessentially
rich, royal, velvety blue”. The crystals have formed where
an underground pool of lava has cooled and solidified into granite,
next to a deposit of limestone. Here, however, the geochemical furnace
operates in a different way from the ruby mines of Burma: the gems
are formed not in the marble but in the granite.
Just as iron-makers
use limestone as a “flux” in a blast furnace, to remove
impurities from the molten iron, so the limestone in Kashmir absorbs
silicon from the molten lava. In pockets of the lava where the silicon
is particularly scarce, the rock solidifies to corundum. Natural
traces of titanium impart its blue colour.
In Montana,
sapphires are created in the same way, where a six-foot wide “dyke”
of lava has cut through limestone. According to one survey, the
Yogo Gulch deposit contains 28 million carats of sapphire, making
it among the largest deposits in the world. These stones are pale
blue, and wonderfully transparent.

The
six-pointed rays of a star sapphire
|
But not all
sapphires and rubies are clear stones. Titanium dioxide (rutile)
may form a form a network of tiny whiskers within the stone. Shine
a light on such a stone and you see a six-rayed star.
Today, it’s
not only Nature that creates gemstones. Small industrial diamonds
can be made artificially; and it’s comparatively easy to manufacture
ruby and sapphire. Artificial gemstones are not just created for
the jewelry market: corundum is an ideal crystal to use in a laser.
When you see the red ray of a laser in a supermarket checkout or
CD player, you are seeing the amplified glow of a tiny gem of pure
ruby.
|
