|
Fuel cells promise to be the environmentally-friendly
power source of the future, but some types run too hot to be practical.
New research may have a solution.
by Patrick L. Barry
Astronauts have been using them for
power aboard spacecraft since the 1960s. Soon, perhaps, they'll
be just as common on Earth - powering cars, trucks, laptop computers
and cell phones.
They're called fuel cells.
By combining hydrogen fuel with oxygen,
fuel cells can produce plenty of electric power while emitting only
pure water as exhaust. They're so clean that astronauts actually
drink the water produced by fuel cells on the space shuttle.
In recent years the interest in bringing
this environmentally friendly technology to market has become intense.
But there are problems: You can't "fill 'er up" with hydrogen at
most corner gas stations. And fuel cell-based cars and computers
are still relatively expensive. These obstacles have relegated fuel
cells to a small number of demo vehicles and some specialty uses,
such as power aboard the space shuttle and back-up power for hospitals
and airports.

Copyright Toyota Motors more
The Toyota
FINE-S, a hydrogen fuel-cell hybrid-electric concept vehicle
revealed at the North American International Auto Show in
January 2003.
|
Now new research is helping to tackle
some of these obstacles. By finding a way to build "solid oxide"
fuel cells that operate at half the temperature of current designs
- 500°C instead of a blistering 1,000°C - researchers at the Texas
Centre for Superconductivity and Advanced Materials (TcSAM) at the
University of Houston hope to make this kind of fuel cell both cheaper
to manufacture and easier to fuel.
"Our key advance was making the heart
of the fuel cell - the sheet of electrolyte that controls the flow
of electrically charged ions - out of a thin film only one micron
thick," says Alex Ignatiev, the director of TcSAM.
In contrast, today's off-the-shelf
solid-oxide fuel cells have electrolyte layers 100 microns thick
or more (a micron is one thousandth of a millimetre). Ignatiev explains:
"The thinness cuts down internal resistance to electric current,
so we can get comparable power output at much lower operating temperatures."
To make this ultra-thin layer, Ignatiev
and his colleagues at TcSAM don't simply shave down a chunk of bulk
material until it's thin enough. Instead, they grow the electrolyte
atom by atom, depositing one layer of atoms at a time in a process
called epitaxy. The thin films in TcSAM fuel cells are about 1000
atoms thick.
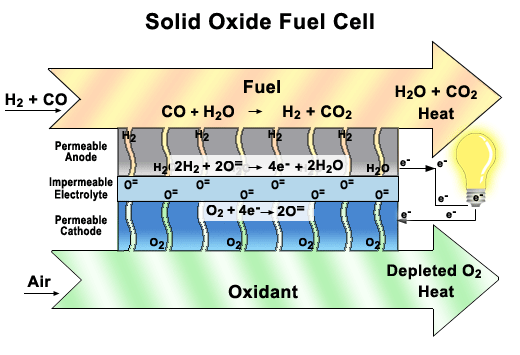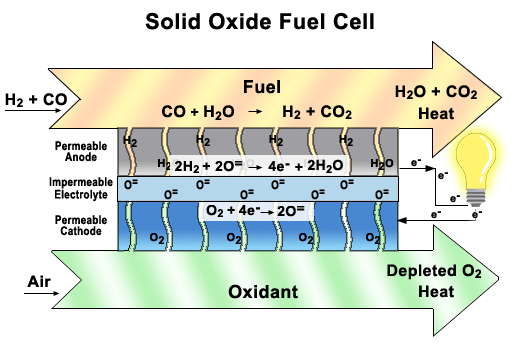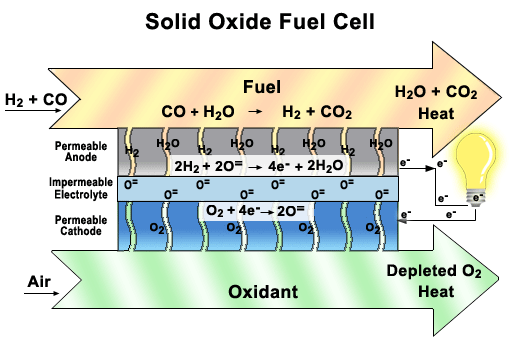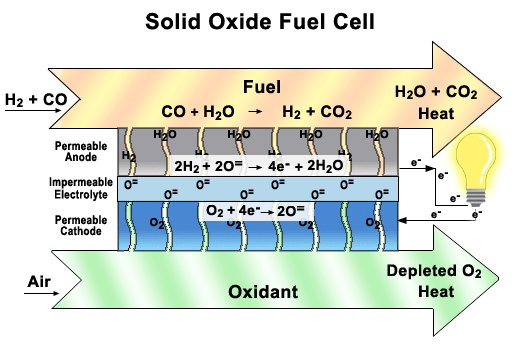
more
A functioning
cell in a Solid Oxide Fuel Cell (SOFC) stack.
|
Squeezing out the same power at half
the temperature creates a domino effect of cost savings. For one,
cheaper materials can be used to build them, rather than the expensive
heat-tolerant ceramics and high-strength steels demanded by 1,000-degree
fuel cells. And the automobiles and personal electronics that could
use these fuel cells can also forgo exotic materials and elaborate
heat-dissipation systems, lowering manufacturing costs. All of this
tips the scales of economic feasibility in the right direction.
Support for fuel cells as the successor
to the internal combustion engine is widespread. All of the major
automobile manufacturers are busily developing fuel-cell vehicles,
and President Bush recently proposed spending US$1.2 billion to
help bring the technology to market.

Copyright 2002 Casio
Inc.
This prototype
Casio laptop can run for more than 20 hours on one refueling
of its fuel cell power supply, shown here removed from the
computer.
|
The portable electronics industry is
also exploring miniature fuel cells as a more powerful, longer lasting
replacement for batteries. Intel, for example, has funded
a start-up company called PolyFuel to develop such a fuel cell for
laptops.
Solid-oxide fuel cells are one of six
types being developed today. Each depends on a different chemical
trick to combine the hydrogen fuel with oxygen to produce power.
The automotive industry is looking primarily at proton exchange
membrane (PEM) fuel cells to power tomorrow's cars,
motorcycles and trucks, but some companies are also considering
the advantages of the solid-oxide variety.
Key among these advantages is the ability
to run on readily available fuels, such as methanol or even gasoline,
which contain hydrogen bound to carbon and sometimes oxygen. The
other five types of fuel cell can do this as well, but only with
the help of additional hardware called a "reformer," which extracts
pure hydrogen from these other fuels. These reformers cost extra
money, add bulk to the engine, and sap power, cutting the engine's
overall efficiency roughly in half.
Solid-oxide fuel cells are able to
consume methanol-like fuels without reformers.
Most of the environmental benefit of
fuel cells is lost when hydrocarbon fuels are used, because extracting
hydrogen from them leaves behind CO2 and pollutant gases
that end up in the exhaust. But it helps solve the "chicken and
egg" problem: Who's going to buy hydrogen-powered cars until most
gas stations have hydrogen pumps? But what company is going to pay
to install hydrogen pumps at hundreds of gas stations until there
are plenty of fuel-cell cars on the road? Solid-oxide fuel cells
can bridge the gap. They can run on methanol or gasoline now and
then switch to pure hydrogen as it becomes available.

Image copyright Powertech Labs. more
Hydrogen
fuelling stations like this one in Vancouver, Canada, are
still rare. The vehicle fuelling up is a Ford FCV.
|
The thin-film variety being developed
at TcSAM improves on this fuelling flexibility. Ignatiev explains:
"Normal solid-oxide fuel cells can use fuels like methanol, but
they become impaired over time as carbon coats the fuel cell's nickel
electrode," he says. "This happens partly because of the cell's
1,000-degree operating temperature. Research shows that this doesn't
happen - at least not to an appreciable degree - at the lower temperatures
at which our cells operate."
TcSAM's fuel cells have not yet been
tested with fuels other than pure hydrogen, Ignatiev says, but the
scientists plan to perform tests with methanol-like fuels during
the next stage of research.
There's still much work to be done.
If all goes well, though, these thin films could pave the way to
clean-running SUV's and other wonders of a hydrogen-based economy.
|
