|
Flames do something odd in space: they form
tiny almost-invisible balls that might reveal the secrets of combustion
here on Earth.
by Linda Voss and Dr Tony Phillips
Paul Ronney wasn't looking for flame balls.
They came as a complete surprise.
It happened in 1984 when Ronney, a combustion researcher,
was at the NASA Glenn Research Centre's Microgravity Drop Tower
in Ohio. He pressed a button and sent a can of burning hydrogen
falling down a 90 ft. shaft. For 2.2 seconds it plummeted, freely
falling and weightless, with a 16mm movie camera recording the action.
Ronney knew that flames did strange things in low gravity - that's
why he was doing the experiment - but he wasn't prepared for what
he saw in the film room later.
The flames had broken apart into tiny balls that
moved around like UFOs. "I thought I had done something wrong,"
he recalled. Some of his colleagues didn't believe him when he described
the experiment. Indeed, "it was ridiculous. No one had ever seen
anything like it."
But the flame balls were real. Later experiments
proved it.
"Flame balls are the weakest flames we have," says
Ronney. "Compared to a birthday candle's 50 to 100 watts, a flame
ball produces only 1 to 2 watts of thermal power. They burn using
very little fuel. It's almost as if a hydrogen-burning flame's last line of
defence as it approaches extinction is to draw itself into a simple
ball."
Ronney, who is now an engineering professor
at the University of Southern California, believes that flame balls
will help him and others crack the unsolved mysteries of burning.
Considering that combustion powers our automobiles, generates our
electricity, and heats our homes, there's much about it we don't
understand. "For example," he says, "a moderate amount of turbulence
makes a flame burn faster, but too much turbulence extinguishes
it." No one knows why.

Tiny flame
balls that form in low gravity are hard to see. These were
filmed in the dark by a low-light video camera onboard the
space shuttle Columbia in 1997.
|
Flames are hard to understand because
they are complicated. In an ordinary candle flame, for example,
thousands of chemical reactions take place. Hydrocarbon molecules
from the wick are vaporized and cracked apart by heat. They combine
with oxygen to produce light, heat, carbon dioxide and water. Some
of the hydrocarbon fragments form ring-shaped molecules called polycyclic
aromatic hydrocarbons and, eventually, soot. Soot particles can
themselves burn or simply drift away as smoke. The familiar teardrop
shape of the flame is an effect caused by gravity. Hot air rises
and draws fresh cool air behind it. This is called buoyancy and
is what makes the flame shoot up and flicker.
Flame balls, on the other hand, are
simple. The balls form in low gravity where turbulence and buoyancy
have little effect. Oxygen and fuel combine in a narrow zone at
the surface of the ball, not hither and yon throughout the flame.
Once ignited and stabilized, their size remains constant. Unlike
ordinary flames, which expand greedily when they need more fuel,
flame balls let the oxygen and fuel come to them. Finally, the fact
that flame balls are spherical reduces their dimension to one: the
radius of the flame itself. "Flame
balls are to combustion scientists what fruit flies are to geneticists,"
says Ronney. "It's not that we want more fruit flies, or flame balls,
but they provide a simple model for testing hypotheses and checking
computer models."
One of many mysteries about fire is the way weak
flames go out before their fuel is totally exhausted. It puzzles
physicists and vexes automakers who want to build clean, efficient
"lean-burning" engines that run on fuel-air mixtures with low fuel
concentrations-much like a flame ball. Ronney believes that studying
one (flame balls) will help us with the other (cars).
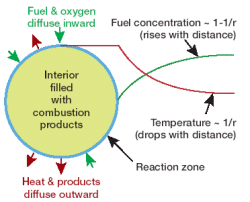
Credit: Paul Ronney.
A schematic diagram of a flame ball.
|
Here on Earth, researchers
can't study flame balls for long. A typical plunge down the drop
tower lasts only 2 seconds. So, working with NASA scientist Karen
Weiland and others at the Glenn Research Centre. Ronney designed
the Structure of Flame Balls at Low Lewis-number (SOFBALL) experiment.
It's a sealed chamber where flame balls flying onboard the space
shuttle can burn for a long time.
SOFBALL orbited Earth
for the first time in 1997 on shuttle Columbia, and it produced
some surprises.
Computer models had predicted
the flame balls would be small and either extinguish or drift into
the chamber walls in a few minutes. Instead they were two to three
times larger than predicted and burned for over 8 minutes until
the experimental system automatically extinguished them. Furthermore,
although the flames were large, they were the weakest ever seen-emitting
little more than 1 watt of thermal power.
The experiment, upgraded
and re-named SOFBALL-2, will soon fly again. It's slated for launch
onboard space shuttle Columbia (STS-107) in late 2002 or 2003. During
the mission, flame balls will be allowed to burn for 25 to 167 minutes.
Instruments will monitor their temperature, brightness, heat loss,
and the composition of their gaseous byproducts. Because flame balls
are so sensitive to motion, the shuttle will drift during the experiments
instead of using its reaction control thrusters to maintain position.

more
A candle
flame on Earth (left) and onboard the space shuttle (right).
|
Because this research
is so fundamental, it touches on many aspects of combustion: lean-burning
engines for cars and airplanes; explosion hazards in mine shafts
and chemical plants; emissions from cars and coal-burning plants;
arson investigations. The list is long ... and it doesn't stop on
Earth.
Flames act differently in space, so
fire safety is also different. If you see a fire on Earth, you might
run over and stomp it out or use a fire extinguisher. In orbit,
rushing over and stomping on a flame might accelerate combustion,
at least temporarily, because you are creating an airflow that did
not exist before. Flames in low-gravity tend to spread slowly, so
stomping might cause a flame to jump to something else when it wouldn't
have otherwise. Furthermore, flame balls are stealthy: they give
off no smoke and little or no visible light. It's hard to extinguish
something you can't find. What happens if a loose flame ball runs
into something? Will it ignite? SOFBALL-2
could answer many such questions.
SOFBALL will also set
the stage for longer-term experiments aboard the International Space
Station inside the Fluids and Combustion Facility - yet to be installed
in the US lab module. That's a long way from Ohio, where Ronney
discovered flame balls in 1984. But he says it's worth the trip
to find out how else "those ridiculous little flame balls" might
surprise us.
|
